Mon, Feb 16, 2026
[Archive]
Nanoreactor-Enabled Formation of Graphitic Film from a Non-Graphitizing Precursor at Low-Temperature
Abstract: (1572 Views)
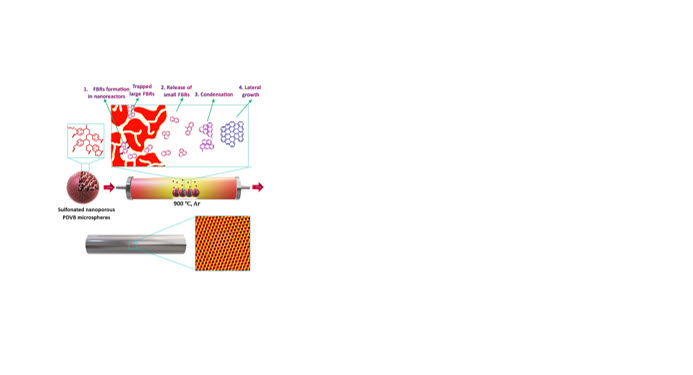
Gas-phase methods for graphite/graphene production, such as chemical vapor deposition (CVD), yield high-quality products but demand catalysts, substrates, high-purity hydrocarbon gases, specialized furnaces, and temperatures exceeding 1000 °C. Here, we demonstrate the synthesis of highly graphitized films with crystalline domains via low-temperature carbonization (900 °C) of nanoporous polydivinylbenzene (PDVB) microspheres, without reliance on a CVD system or catalysts. The films formed on the inner surface of the furnace quartz tube and were characterized by Raman spectroscopy, Fourier Transform Infrared (FTIR) spectroscopy, X-ray Diffraction (XRD), and high-resolution transmission electron microscopy (HRTEM). Raman spectrum revealed a high graphitization degree (ID1/IG = 0.78), surpassing reported values for catalyst-free plasma- or low-pressure-assisted CVD. XRD showed a sharp diffraction peak at 2θ = 26.37° (d = 3.37 Å), exactly matching the (002) plane of Graphite-2H, while HRTEM and selected area electron diffraction confirmed crystalline domains with p63/mmc symmetry. We propose that the intricate network of nanopores as nanoreactors in PDVB microspheres enables the generation and controlled release of fused benzene rings into the quartz tube, where they condense to form crystalline films. This approach reveals how a nanoscale confinement can be translated into a macroscopic, scalable route, offering a low-cost and facile method for graphite or graphene production.
Type of Study: Research Paper |
Subject:
Ceramic Materials and Engineering
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |